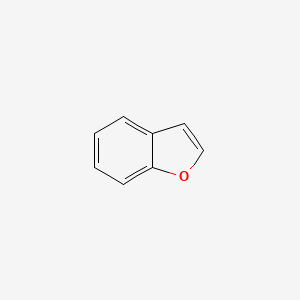
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.
Laboratory methods
Benzofurans can be prepared by various methods in the laboratory. Notable examples include:
- O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation.
- Perkin rearrangement, where a coumarin is reacted with a hydroxide:
- Diels–Alder reaction of nitro vinyl furans with various dienophiles:
- Cycloisomerization of alkyne ortho-substituted phenols:
Related compounds
- Substituted benzofurans
- Dibenzofuran, an analog with a second fused benzene ring.
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Benzothiophene, an analog with a sulfur instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
References